Abstract
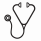
Background: Cancer is the second leading cause of death in Mexico among children 5-14 years. In 2017, Mexico in Alliance with St. Jude (MAS), a multi-site intersectoral collaboration, explored reasons for suboptimal outcomes for children with acute lymphoblastic leukemia (ALL). Results showed 82% of patients to be classified as high-risk and 30% of patients be missing standard molecular or minimal residual disease (MRD) studies needed to inform risk- group stratification. Making sense of the molecular characteristics was challenging in the context of variable access and lack of common denominators. These findings led to the development of the "Bridge Project"; a prospective quality improvement demonstration project aiming to bridge this access and quality gap through multi-site collaboration, centralization, and standardization.
Methods: Six MAS collaborating hospitals, located in six different states (Mérida, Guadalajara, Sinaloa, Baja California, Chiapas, and Veracruz), have sent diagnostic samples for children 0-18 years old with suspected ALL to Hospital Infantil Teletón de Oncología (HITO), in Queretaro, which serves as the centralized laboratory for the MAS cooperative group. Access to the diagnostic panel is secured upfront through dedicated funding obtained from a local foundation. The first sample was shipped in July 2019. The consensus-derived diagnostic panel includes morphology, immunophenotype, karyotype, fluorescent in situ hybridization (FISH), molecular biology (RT-PCR) and flow cytometry (FC) MRD at two time points. Protocols vary by institution and MRD evaluation is only done if the institutional protocol incorporates MRD-evaluation into the risk-group stratification. Hospitals send several empty boxes prior to shipping patient samples to assess their regional shipping vendors and timelines. The also standardize data collection processes and use PDSA cycles to monitor sample quality and address issues with sample quality. HITO produces FC results (for diagnosis or MRD evaluation) and FISH/cytogenetic results within 3 to 21 days of sample arrival.
Results: As of July 2021, the centralized lab has received samples from 217 patients with suspected ALL through this project and 93% of these samples have arrived within the 48hr target. Of these, 176 (81.1%) had confirmed ALL and 14 (6.4%) had acute myeloid leukemia (AML), 2 (0.9%) cases had other malignant conditions, and in 25 (11.5%) of the cases leukemia was ruled out. Among the 176 cases of confirmed ALL, 162 (92%) had B-cell lineage and 14 (8%) had T-cell lineage. FISH was reported for 172 (97.7%) patients and positive in 126 (73.2%) cases; 46 (26.7%) cases had reported gains, 34 (19.7%) detected t(12;21), 18 (10.4%) detected iAMP cr21, 8 (4.7%) detected breaks of the MLL gene t(4;11), 7 (4%) detected t(1;19), and 6 (3.4%) detected t(9;22). Four T-cell ALL cases had breaks CDKN2A del(9)(p21) and three cases had TRA/D rearrangement (14) (q11.2). Karyotype alterations were detected in 90 (52.3%) of the samples, of which 41 (23.8%) showed hyperdiploidy and 13 (7%) showed complex karyotype. One case of hypodiploid was identified. Day 15 MRD was assessed in 131 (80%) patients with B-cell lineage, Day 84 MRD was assessed in 99 (61.1%) patients with B-cell lineage, and Day 29 MRD in 8 (57.1%) patients with T-cell lineage whose treatment schema utilizes MRD-based stratification. Among B-lineage patients, 84% had MRD <1% at Day 15 and 94.9% MRD <0.01% at Day 84. Among T-cell lineage patients, 75% had MRD <0.01% at Day 29 and 80% MRD <0.01% at Day 84, but numbers for T-cell lineage were small.
Conclusions: Given proper structural and financial supports, patients with suspected childhood ALL in Mexico can access a comprehensive diagnostic panel following a centralized laboratory approach. Preliminary results from the Bridge Project allow characterizing childhood ALL to a degree that has not been previously possible in Mexico. In this cohort, favorable characteristics such a t(12;21), gains, and hyperdiploidy are observed in frequencies similar to those reported in Hispanic cohorts in high-income countries. MRD results are for B-cell lineage are also consistent with the literature. Continued engagement in this project from hospitals in diverse geographic settings and with diverse patient populations will allow the MAS cooperative group to continue to improve the characterization of childhood ALL in Mexico.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract